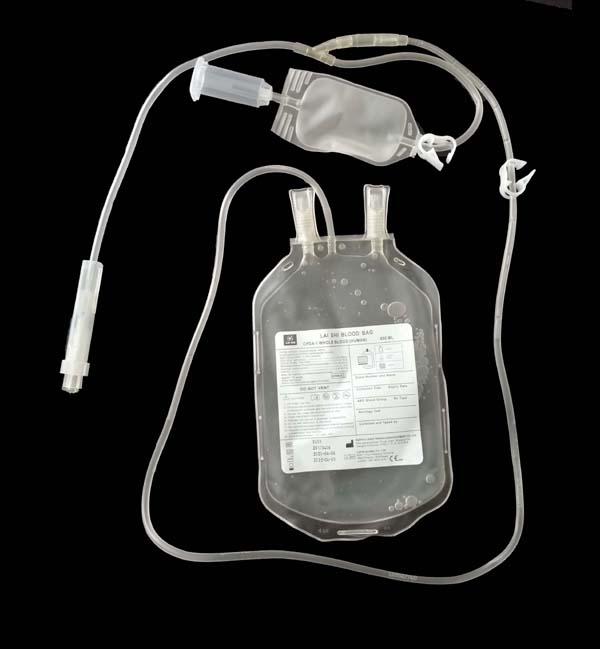
Model No.︰S450C
Brand Name︰LAISHI
Country of Origin︰China
Unit Price︰US $ 0.7 / pc
Minimum Order︰30000 pc
The single blood bag system has different configuration.
• Sterile, pyrogen free, non toxic , ensure external sterility of the bags,
• Slit present at the bottom of the bag is satisfactory to hang the blood bag during transfusion.
• The plastic blood bag has a shelf life of minimum 2 years.
• Packing of goods: Individual blood bags are packed in a plastic pack and a maximum of 5 bags is packed in aluminum foil pack. These foil packs are packed in the corrugated boxes which indicates clearly and legibly the manufacture name,product name, batch number, quantity, manufacturing and expiry date, gross and net weight and consignee’s name address.
• Storage of human blood and collection of blood volume is 250ml, 350ml, 450ml, 500ml.
| Code | Anticoagulant | Size | Needle | Rem |
| S250C | CPDA1 35ml | 250ml | 16G needle | Optional NP, SP, CT |
| S350C | CPDA1 49ml | 350ml | ||
| S450C | CPDA1 63ml | 450ml | ||
| S500C | CPDA1 70ml | 500m |
DESIGN
• No risk of contamination and airembolism [ closed system ] with all leak proof seals.
• Slit on both sides of the bags are enough to accommodate 5-10 ml volume test tubes.
• The capacity of the bag is enough to prevent any rupture of the bag from the seam when it is filled to the requisite volume of blood.
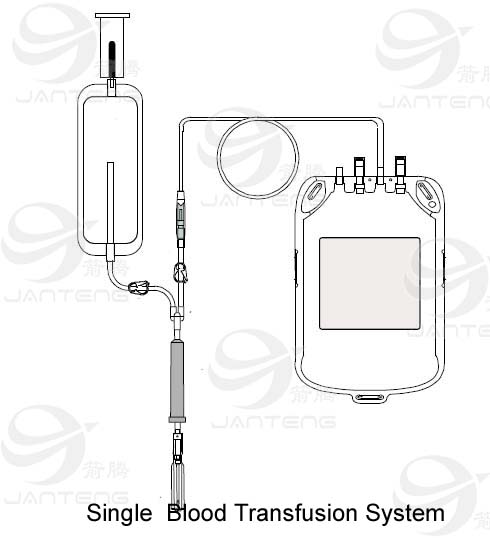
TUBING
• Flexible and non kinking
• Non sticking
• Transparent
• Leak proof
• Tubing has same ID/segment number as that on the bag
• The tubes has multiple printed ID/segment numbers

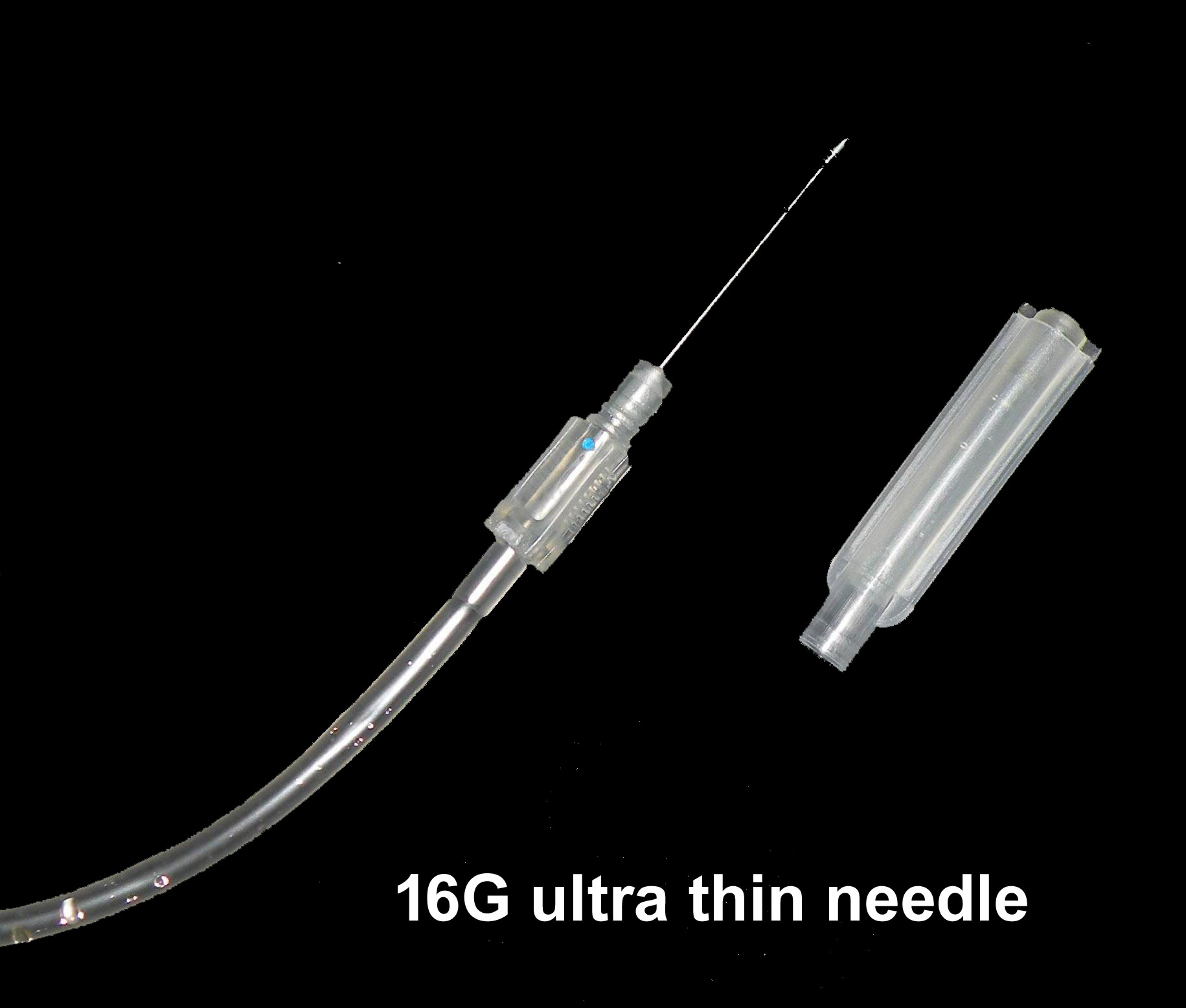
NEEDLE
• 16 gauge short bevel angled
• Siliconized for maximum donor comfort
• Rust proof
• Tightly fixed with hub covered with sterile guard
• Hermetically Sealed
EXTERNAL PORT
• Tamper proof and shouldn’t be recapped
• Easily accessible
PACKAGING
• Protective dual packaging [individual and aluminum] eliminating microbial contamination on surface maintaining thecontents of the bag.
• Easy to handle.
ANTICOAGULANT
Each 100 ml CPDA-1 [U.S.P] contains:
- Citric Acid (anhydrous)……………………………………….......… 0.299g
- Sodium Citrate (dehydrate)……………………………….......…..2.63g
- Monobasic Sodium Phosphate (monohydrate)…......…..0.222g
- Dextrose (monohydrate) ……………………………….........……… 3.19g
- Adenine ……………………………………………………………..............…..0.0275g
Water for injection, sufficient quantity to make……………100ml
• Clear And colorless
• No discoloration on storage at roomtemperature
• Manufacturer to provide anticoagulant qualitycheck certificate.
LABEL
• Non peel off
• Heat sealed labels
• Remain attached between room temperature to80 c with a transparent adhesive
• Label has printed manufacturing and expiry date and lot number of the bag
• Expiry date 2 years from the date of sterilization
• Label provides sections for recording donornumber, collection and expiration date of blood, blood group and Rh factor,Serology test results and signature of attendant.

RESISRANCE TO DISTORTION
• When filled to normal capacity the bag is able to with stand accelerate of 500g for 30 mins at temperature 40 to 24 ℃ without becoming permanently distorted or rupture.
• bags are able to with stand temperature up – 80℃ without breakage.
The blood bag manufacturing site has been engaged in the manufacturing since 1986, it has about 260 well trained workers and a strong professional technical and quality team. With modern and strict management, Laishi has became a reliable brand supplier for transfusion system in world transfusion industry. All the products have passed strict TUV & GMP authentication system .
Payment Terms︰ TT / LC